By Franziska Kersten, PhD Candidate in Marine Geology and Paleontology – Alfred Wegener Institute for Polar and Marine Research, Bremerhaven (Germany).

Ocean corals are threatened by ocean acidification. Photo from yaleclimatemediaforum.org
The 2007 IPCC (Intergovernmental Panel on Climate Change) Synthesis Report discusses ocean acidification and its potential to harm marine calcifiers (e.g. corals) in the future. Yet a recent report in Science unequivocally states that parts of the ecosystem are already affected by the changes in ocean chemistry that result from anthropogenic carbon dioxide emissions (Service, Science 2012).
A decrease in ocean pH from 8.2 to 8.1 has been observed since pre-industrial times. The pH scale is logarithmic, so a drop of 0.1 pH units, though it might seem small, is equivalent to a 30 percent rise in ocean acidity. According to IPCC projections, ocean pH will be reduced by a possible 0.35 units through the end of this century, with profound effects on marine calcifying organisms and the carbon cycle.
On such (geologically) short timescales, the carbon cycle is mainly driven by the exchange between the atmospheric and oceanic reservoirs via the solubility and biological pumps.
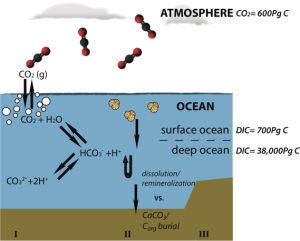
I Equilibrium reactions of CO2 in seawater, II simplified sketch of the biological pumps, III pre-industrial capacities of dissolved inorganic carbon (DIC) reservoirs (values from Sigman and Boyle, 2000). Image by Franziska Kersten.
Carbon dioxide (CO2), which, next to water vapor is the most important greenhouse gas in our atmosphere, readily dissolves in water. In the surface ocean, CO2 reacts with water to form (primarily) bicarbonate, carbonate and hydrogen (H+) ions, thus lowering the pH (pH=-log[H+]) (see Figure below). This process is part of the solubility pump mechanism, which mediates the exchange of CO2 between atmosphere and surface ocean. CO2 is more soluble in cold and fresh water than in warm and saline water, accordingly the uptake of anthropogenic carbon differs between regions.
The biological pumps regulate the exchange of carbon between the atmosphere and surface ocean as well as its interior. In the surface ocean, CO2 is consumed by phytoplankton through photosynthesis, leading to a drawdown of CO2 from the atmosphere by the organic carbon pump. The calcium carbonate (CaCO3) counter pump works in the opposite direction. Calcification of shell-building organisms consumes (mainly) calcium and (bi-)carbonate ions, and releases CO2. Post-mortem, both CaCO3 and the organic material is exported away from contact with the atmosphere and into to the ocean interior. Here it is either preserved in the deep ocean carbon reservoir or dissolved/remineralized and brought back to the surface and into contact with the atmosphere. The dissolution of CaCO3 leads to an increase in bicarbonate and carbonate ions, thus lowering atmospheric CO2 in upwelling regions, while the organic carbon that is set free during remineralization leads to CO2 degassing. The globally averaged ratio of organic to inorganic carbon export is 4:1, maintaining equilibrium between CO2 drawdown and outgassing. However, a declining ocean pH that harms CaCO3-shell building organisms and their dependent species has the potential to critically affect this ratio.
The Science article mentioned above highlights some present-day examples of this ongoing process. An oyster larvae hatchery on the Oregon coast reportedly lost 80 percent of its annual production in 2007 and 2008 due to ocean acidification. Under certain conditions, CO2 enriched deep-water reaches the surface, reducing the pH in this area and thus the carbonate ion concentration that the oysters and other shellfish in the hatchery use to build their shells. As a result, most of the larvae are either killed or their growth severely inhibited. Amongst other members of the food chain, photosynthetic algae—representing potential carbon sinks—also show clear concerning signs of change. A drop in shell weight has been reported in certain coccolithophore and foraminifera species, coeval with the rise in atmospheric CO2. The examples go on and it is clear that we are just beginning to experience how climate change is affecting marine ecosystems.
References
IPCC, 2007: Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri, R.K and Reisinger, A. (eds.)]. IPCC, Geneva, Switzerland, 104 pp.
R.F. Service: Rising Acidity Brings an Ocean of Trouble.Science 13 July 2012, Vol. 337, pp. 146-148
D.M. Sigman, E.A. Boyle: Glacial/interglacial variations in atmospheric carbon dioxide. Nature 19 October 2000, Vol. 407, pp. 859-869

Comments are closed.